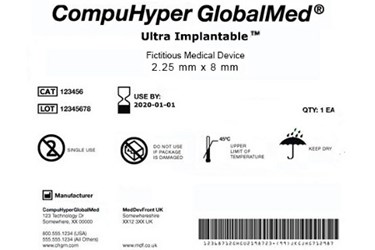
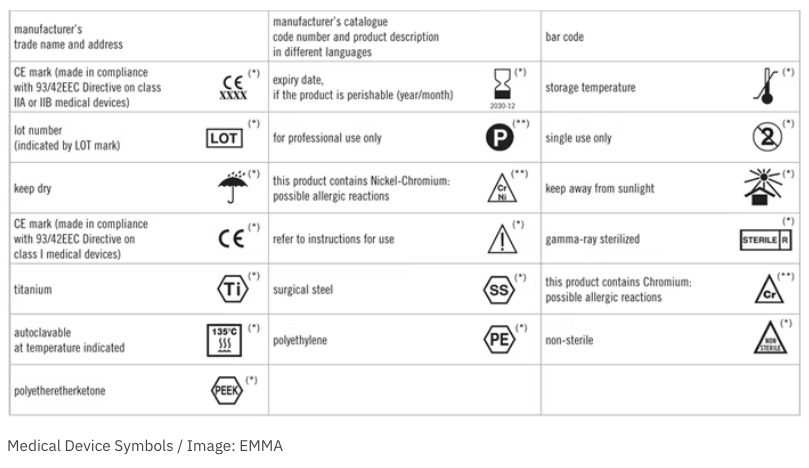
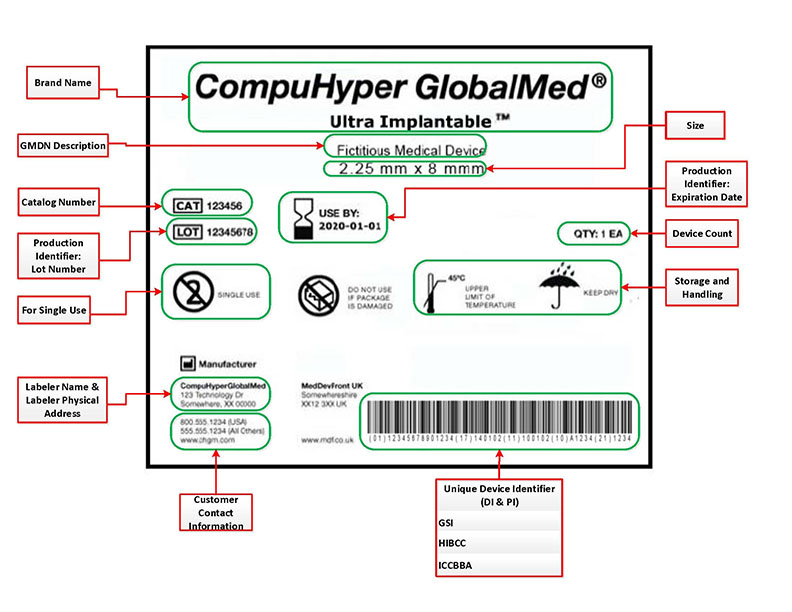
45 fda guidance use of symbols on labels
Understanding Food Labels | The Nutrition Source | Harvard T.H. Other potential allergens include gluten and color additives such as FD&C Yellow No. 5. The FDA mandates that a product containing FD&C Yellow No. 5 must identify it on the food label. The term “gluten-free” can be listed on a label if it meets a specific maximum amount of gluten as defined by the FDA. Sell-by, Best-by, and Use-by dates Summary: Use of Symbols in Labeling (Final Rule) - FDA 26 Mar 2018 — The final rule also specifies that the use of symbols, accompanied by adjacent explanatory text continues to be permitted. FDA is also revising ...
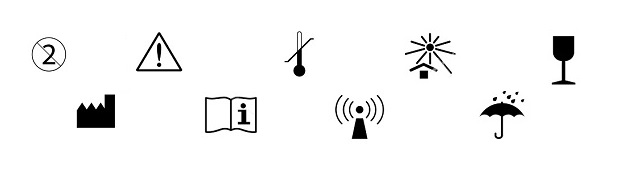
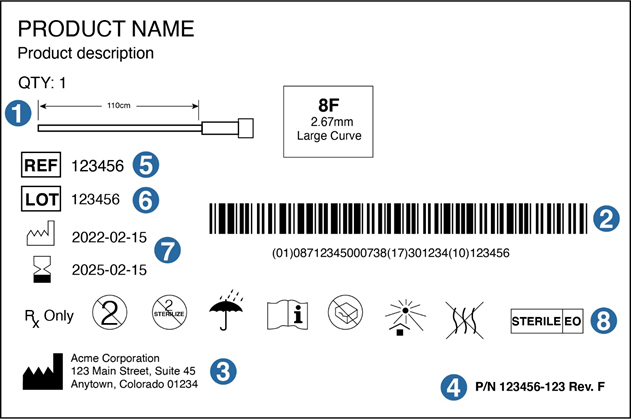
Medical Device Labeling New ISO 15223-1 FDA Guidance ... 16 Aug 2021 — The guidance further states that the FDA does not require a specific type of symbol to disclose the presence of AIDC technology containing the ...

Fda guidance use of symbols on labels
IFU for Medical Devices, a Definitive Guide (EU & US) - INSTRKTIV Use of Symbols - 21 CFR Part 801.15; In Vitro Diagnostic Products - 21 CFR Part 809; ... Although not mentioned in the FDA Guidance document, ... in the Netherlands, labels and instructions for use must be in Dutch. This requirement is contained in Article 6 (2) of the Medical Devices Decree ... Use of Symbols in Labeling - Federal Register 15 Jun 2016 — FDA has generally interpreted existing regulations not to allow the use of symbols in medical device labeling, except with adjacent English- ... Use of Symbols in Labeling: Frequently Asked Questions - FDA 22 Mar 2018 — Yes. The final rule explicitly allows for the optional use of stand-alone symbols in medical device labeling under certain circumstances. We do ...
Fda guidance use of symbols on labels. Using Symbols to Convey Information in Medical Device ... - FDA 19 Jul 2018 — In June, FDA issued the Use of Symbols in Labeling final rule, which describes the circumstances in which manufacturers can use a ... The Joint Commission issues new Quick Safety advisory on … 28.6.2022 · Manufacturers of certain medical devices and products must include labeling on or with their devices, according to the U.S. Food and Drug Administration (FDA). The Joint Commission requires that organizations follow the manufacturer’s written instructions for use (IFU) to ensure the end-user understands how to use, clean, disinfect, reprocess and store … Will new FDA final rule on symbols for device labels make ... 3 Aug 2022 — In June, FDA finally dropped their requirement for symbols for device labels to be explained with adjacent text and now allows the use of ... Child-Resistant Packaging Statements in Drug Product Labeling This guidance is intended to assist applicants, manufacturers, packagers, and distributors (collectively referred to as firms) who choose to include child-resistant packaging (CRP)
Use of Symbols in Labeling | FDA The final rule would provide medical device manufacturers with the option to use symbols established in a standard developed by a standards development ...34 pages Use of Symbols on Labels and in Labeling of In Vitro ... 28 Oct 2003 — Guidance for Industry and FDA. Staff. Use of Symbols on Labels and in. Labeling of In Vitro Diagnostic. Devices Intended for Professional.12 pages Device Labeling | FDA - U.S. Food and Drug Administration 23.10.2020 · Introduction to Medical Device Labeling Label vs. Labeling. The U.S. Food and Drug Administration (FDA) develops and administers regulations under authority granted by laws passed by Congress that ... Use of Symbols in Labeling: Frequently Asked Questions - FDA 22 Mar 2018 — Yes. The final rule explicitly allows for the optional use of stand-alone symbols in medical device labeling under certain circumstances. We do ...
Use of Symbols in Labeling - Federal Register 15 Jun 2016 — FDA has generally interpreted existing regulations not to allow the use of symbols in medical device labeling, except with adjacent English- ... IFU for Medical Devices, a Definitive Guide (EU & US) - INSTRKTIV Use of Symbols - 21 CFR Part 801.15; In Vitro Diagnostic Products - 21 CFR Part 809; ... Although not mentioned in the FDA Guidance document, ... in the Netherlands, labels and instructions for use must be in Dutch. This requirement is contained in Article 6 (2) of the Medical Devices Decree ...















![PDF] Signs of change or clash of symbols? FDA regulation of ...](https://d3i71xaburhd42.cloudfront.net/103393b061f49e3976a4eaa8b35a160bfa9b2f8a/28-Figure3-1.png)








Post a Comment for "45 fda guidance use of symbols on labels"